

新闻中心
Registration of Medical Devices — Official Statistics for 2018 Published
On April 25, the Federal Service for Surveillance in Healthcare (Roszdravnadzor) held a board meeting to summarize its performance in 2018, including the release of data on the registration of medical devices. Since Government Decree No. 1416 entered into force in 2013, the number of registered devices has varied highly from year to year, as we mentioned last year at the Raifarm Conference on September 25
The published Roszdravnadzor data is available in the Service’s video (https://youtu.be/VYQOQS7_VvY).
Screenshot from the Roszdravnadzor video presentation
In 2018, a total of 1,342 medical devices were registered, which is 61 devices or 5% less than in 2017. In 2017, the number of registered devices was 62 devices (4.23%) lower than in 2016. The trend in the number of registered devices is decreasing (note the trend line in the chart below). In this case, the trend line is approximate and reflects the prognosis for the next period.
The video does not mention the causes of the decline in the number of marketing authorizations issued, but it can be assumed that the increasing complexity of regulatory requirements is a major cause. Expert requests to provide documentation and information continue to grow in complexity, and require considerable efforts to prepare a comprehensive response.
The requests focus primarily on technical, operational, and regulatory documentation, which must provided with the utmost precision. Inconsistencies in the technical documentation provided by manufacturers are another reason for issuing requests. Applicants have to spend considerable time looking for such inconsistencies, which can happen for many different reasons. The most common causes are simple typos or cases when manufacturers supplied a document for a previous version of the medical device. Experts also frequently issue requests for device labeling and packaging, and also require stability and aging test results for devices with an expiration date. Regulatory specialists are required to have an ever-increasing knowledge of this field and learn to navigate the tangled web of bureaucracy in registration-related legislation.
Screenshot from the Roszdravnadzor video presentation
The domestic/foreign ratio for medical devices is 60%/40%, respectively. In absolute numbers, this equals 805 registration certificates issued for domestic products and 537 certificates for imported products. No information is provided in the video on the numbers of medical devices registered per risk class.
In 2018, according to the official Roszdravnadzor database, a total of 336 registration certificates (25% of the total number of certificates) were issued for foreign and domestic Class I devices, 517 registration certificates (39%) for Class IIa devices, 336 certificates (25%) for Class IIb devices, and 153 certificates (11%) for Class III devices.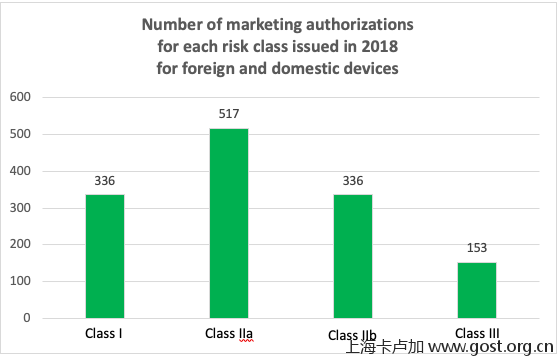
A 38% decrease in the number of registration denials in 2018 compared to 2017 is also noteworthy, although this is likely determined by the fact that many registration dossiers are returned to applicants at the first stage of examination before reaching the “substantive examination” procedure, rather than the efficacy of Roszdravnadzor’s new “simplifications.” For example, we may recall some applicants’ recent complaints regarding requests to provide license agreements for trademarks, which in some cases is simply impossible. The situation is the same with requests related to particular aspects of devices and the economic activity of companies, which confuse many foreign manufacturers. In both cases, the registration process reaches a dead end and the application file is returned to the applicant. Returning documents in this manner is not considered a registration denial.
The chart below shows the number of marketing authorizations issued from 2007 to 2018 broken down by domestic and foreign devices. The green line indicates when Government Decree No. 1416 On the Approval of the Rules for State Registration of Medical Products entered into force. For 2017, the overall number of marketing authorizations, both domestic and imported, is indicated. A total of 1,403 marketing authorizations were issued.
Overall, although the registration procedure for Class I medical devices and IVD was simplified in 2018, the regulatory environment related to registration still remains complex.
Author:
Mikhail Vinogradov
Head of Regulatory Affairs (Medical Devices)
Image:
俄罗斯医疗器械注册认证程序和要求

上海经合工业设备检查有限公司
021-36411223 021-36411293
邮件:gost@gost.org.cn gost-r@163.com
skype:gostchina
微信:18621862553



微信号 扫一扫联系我 公众号 扫一扫 企业微信
联系我们
俄罗斯标准测试认证中心 ООО "Стандарт-Тест"
海关联盟授权代码:RA.RU.11AB24
中国代表处:上海经合工业设备检测有限公司
gost@gost.org.cn eacchina@cu-tr.org
0086-021-36411293 021-36411223

快速链接 版权所有上海经合工业设备检测有限公司 沪ICP备10027014号-16 沪公网安备31011502402895号
网站内容和图片版权所有,未经允许,不得转载复制拷贝和使用 俄罗斯医疗器注册 欧亚联盟药品查询
版权所有 © 上海经合工业设备检测有限公司 保留一切权利。俄罗斯计量证书查询 欧亚联盟EAC证书查询


